Research
Research Group
The interview with staff members of Professor Ozawa’s laboratory
Tiny Saviors protect the global environment
We’ve discovered the nanoparticle that purifies the automobile emission, as well.

We often hear of nanoparticles these days. In Professor Ozawa’s laboratory, they specialize in the ways of protecting the environment with nanoparticles. For the purification of the environment, devices like air cleaners are put in a variety of places and clean the air thoroughly. However, what and how are they doing?
I asked staff member of the laboratory.
Can “Core-shell type CeO2-ZrO2” reduce Pt content in exhaust catalyst?
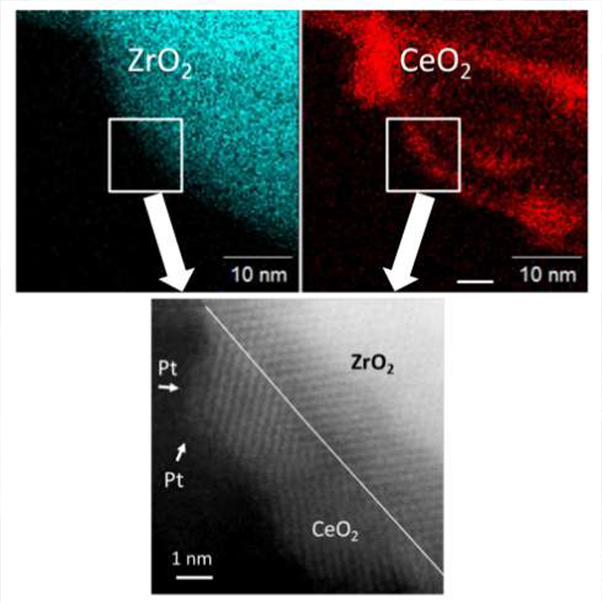
The TEM image of PT/CeO2/ZrO2 nanoparticle catalyst and the chemical composition analysis of Ce and Zr
・ The size of Pt particles is about one nanometer.
・ CeO2, which is about five nanometers, is purifying harmful gases.
The striped pattern shows the crystal lattice of Ce-O-Ce in the CeO2 nanoparticle.
We are making researches with a view to decreasing air pollution.

Professor
Masakuni Ozawa
profile
He graduated from the department of Engineering at Nagoya University in 1979, and after receiving a MA. at the same university, was employed at Toyota Central Research Labs.Inc., where he worked at the factory by using electron microscopes for fault analysis.
When he reached a certain point in his research, auto exhaust catalysts were invented and put to practical use. Then he made fresh start in his research. and in 1993, was employed as associate professor at the lab of Nagoya Institute of Technology. Later. he worked at a research facility located in Tajimi City, Gifu Prefecture, and became the director of the facility. In 2016, he returned to Nagoya University and has been in the present position since 2016. He says he has no hobby now though he composed tankas in his youth. His pleasure is to have a drink at dinner enjoying vegetables he grows in his family garden.
I hear this lab is for making researches with a view to cleaning motor exhaust.
Ozawa:We’re making researches to protect the global environment, particularly to decrease air pollution. In order to achieve the purpose, it’s necessary to reduce the amount of exhaust gases from factories and automobiles. About many cases of fundamental research, we often say, “Wonderful, but it will take dozens of years before they are put to practical use.” As you may know, I worked for more than ten years in a business company. That may be the reason why I want to conduct such practicable researches as we can use soon. We not only try to clean automobile exhaust, but also aim higher at making the device more efficient, a lot easier to use, and more inexpensive.
How do you clean automobile gases?
Ozawa:The main method of ours is to cause chemical reactions with the catalyst and change such noxious gases as CO, HC, and NOx*1 into such innoxious gases as CO2, H2O, and N2. This is called a three-way catalyst*2. It was developed to comply with the new exhaust restrictions that started about 1970. We fit this nanoparticle*3 three-way catalyst to the ceramic honeycomb that is durable to sudden changes of temperature and strong vibrations. The honeycomb*4 is put into the automobile exhaust pipes to clean the gases. It is used in most automobiles including HVs.
You’re trying to improve the purification capacity, aren’t you?
Ozawa:Yes, we are. The catalyst to clean exhaust gases mainly uses precious metals like Pt. Pt is rare and expensive. As a catalyst promoter*5, we use another rare earth metal, Ce. Since these metals are indispensable for our industry and important natural resources, it is absolutely necessary not to waste them and to develop alternative resources. We developed Ceria Zirconia in 2013. It works as a catalyst promoter*6 and enables us to reduce the amount of Ce by more than 30 precent. We take this as a turning point, and are trying to develop nano materials that work as effectively as possible.
The beauty of chemistry lies in the moment when one thing changes into something else.
Why did you direct your attention to cerium out of so many chemical elements?
Ozawa:The oxide of Cerium, CeO2, turns dark yellow when oxidized, and blue when deoxidized. It changes from blue to yellow, and vice versa. It is an artistic technology, and beautiful. That’s why I set my eyes on it. Physics is a beautiful science itself, but I think the beauty of chemistry, particularly in case of materials, lies in the moment when one thing changes into something else.
You seem to have found great ambition in the science of Chemistry.
Ozawa:Look at this ceramic (shown in the photo) made by a native American. The smooth part is black and the rough part brown. It was reddish brown clay with red iron oxide in it, and when it was baked, it was deoxidized and turned deep-black. The rough part is brown because it was exposed to the air and oxidized. The color (or the state) of the same thing can be different. The same is true of jewels. (He laughingly said) Here are stones that could be jewels. As a child, I thought they were beautiful jewels.


The Left photo:a traditional ceramic made by a native American.
The right photo:jewels (that could be made from stones).
Ozawa said, “Even in my childhood, I wondered why there were stones of such a variety of colors. Oxidation-reduction changes the colors of ceramic and stones, and the colors depend on the metals contained.

The catalyst as the substitute for precious jewels

Assistant Professor
Masatomo Hattori
profile
In 2009, he received the doctorate of material engineering at graduate school of Nagoya Institute of Technology, and in the same year, was employed as doctoral research fellow at the Institute of Catalytic Organic Chemistry of University of Poitiers in France. In 2011, he was employed as designated assistant professor at the Ecotopia Science Institute of Nagoya University. In 2013 he was employed as designated assistant professor at Advanced Ceramics Research Center of Nagoya Institute of Technology. In 2016, he returned to Nagoya University and worked as designated assistant professor at IMaSS, and in 2017 became assistant professor and has been the same ever since. His likes and hobby: He is a member of an amateur orchestra and plays the contrabass. He likes to sing at karaoke bars, and also likes to go for a drive.
Mr. Hattori, when did you become interested in chemistry?
Hattori:Since my boyhood, I have been fond of assembling parts into a complete unit. As a young boy, I used to play with cardboard boxes or milk cartons. Though I was a poor reader, I was good at mathematics. In the subject, I could frame answers for myself with the knowledge I had. In the meantime, though I didn’t know why, I found myself enchanted with various chemical reactions of substituents of benzene rings when I was writing them in my notebook.
Do you mean that benzene rings determined your fate?
Hattori:When I was writing chemical reactions of substituents of benzene rings in my notebook, I felt as if I were trying to solve puzzles. I used to think of chemical reactions as characters, but the moment I started to regard them as images, I was enchanted with them. I became hooked on them. That was when I was captivated by chemistry. Later, when I had an opportunity to talk with some professors at the college entrance orientation, I started to think of doing something to solve the global warming and the air pollution through the study of chemistry.
What kind of research are you carrying on now?
Hattori:The research I am conducting now is about the catalyst to clean exhaust gases emitted from various combustion engines including automobile ones. So far, precious rare metals like Pt have been used because their purification capacity is so high. I am trying to find an alternative thing whose purification capacity is as high or higher than Pt.

The main work I’m given is computation. It’s my field of expertise.

The photo was taken when she was a JSPS Research fellow
Assistant professor
Maki Nakamura (as of 2021)
profile
She dreamed of space development. She studied physics and received a doctorate of Science at the University of Tokyo.
However, she changed her course in life from space development and started to research in the field of automobiles that interested her. She was employed at Tokyo Institute of Technology, and later employed as a research fellow at IMaSS. She gave birth to her first and second child, and is an assistant professor now.
Her likes and hobby: She likes to drive. She complains that there are very few manual transmission cars now. She likes diesel cars. (She laughingly said) They’re amazing! She started to be interested in automobiles when she was about three years old. The first car she liked was a MAZDA SA22C. She is a sportswoman and she is interested in now are bowling and boxing. She likes to dance to music together with her children.
Dr. Nakamura, may I ask you what research do you conduct?
Nakamura:I usually deal with particulates. I carry on the research on the filter called “DPF” (diesel particulate filter) to remove soot, or particulate matter, from the exhaust gases of a diesel engine. I simulate and quantify the process by which fine particles attach to the filter, and the process by which they aggregate*7. Another thing I do in my research is to carry out computations about the honeycomb. The material for the honeycomb has lots of microscopic holes as large as about 300 micrometers*8. Its wall has lots of holes. I compute, for instance, to find what the flowing is like inside the holes and where the catalyst works best.
Do you find it difficult to carry both your career and raising your children?
Nakamura:I have two children. One is one year old, and the other two years old. Certainly, I’m very busy taking them to and from the day nursery. I have learned to focus my concentration on doing things in a limited time. I sometimes failed to be in time for the seminar held once a week, or had to leave it halfway, but Professor Ozawa has a big heart and doesn’t mind it at all. The main work I’m given is computation. He knows well that it’s my field of expertise.
The research for protecting the global environment
When did you start to show interest in environmental problems?
Ozawa:As a young boy, I lived in the suburbs of Nagoya City. It was the countryside full of nature. I remember that there was a hill near our house and that we could find natural hexagonal-prism crystal there. At junior high school, I did carbonization experiment for myself. It had such a strong impact on me that I wanted to study science, especially chemistry. In those days, however, pollution was already increasing. In this neighborhood, Yokkaichi asthma from factory smoke, exhaust gases on the national route 1 and 23, air pollution caused by burning coal. In fact, I had mixed feelings like this. “I am interested in the problems, but it seems that I’m doing something wrong.” With my feeling of uncertainty, I belonged to the Astronomical Observation club at senior high school, and in college, I composed poems for fun, though I majored in technology.
How did you put away the feeling of uncertainty?
Ozawa:I thought of making crystal like jewels for my graduation thesis, and went to Synthetic Crystal Research Laboratory, which became part of IMaSS later. What I found there were the method in which the ultra-high voltage was used for producing crystal, and the method of hydrothermal synthesis in which crystal was made in water. At that time, I asked if a pressure vessel was used because the stone was so hard, and then the reply was that the stone was so soft. I heard that crystal was produced by means of the pressure in the earth. I also heard that crystal was produced in the vessel changing its form properly. That changed my way of seeing things. That was when I thought I understood something of science.
Fundamental knowledge
*1 NOx
The term for Nitrogen Oxides. It often refers to NO and NO2. It is produced when nitrogenous compounds contained in the fuel and nitrogen in the air are oxidized in a fire. It is also emitted as car and factory exhaust. It causes photochemical smog, acid rain, and the like.
*2 Catalyst
Generally, it is a substance that makes a chemical reaction happen faster without being changed itself.
*3 nanoparticles
Nano is one billionth part of a unit. One nanometer is one billionth of one meter, and one millionth of one millimeter. It is about 100,000 times smaller than the diameter of a human hair.
The characteristic of nanoparticles: if the particles of the same weight are compared, the gross area of the surface becomes larger as their size becomes smaller. That means that the gross area of a large number of tiny particles are far larger than that of a big particle.
About catalytic reactions: Since catalytic reactions take place on the surface of the catalyst, reducing the catalyst particles to nanoscale size, i.e., nanoparticles, greatly accelerates the reaction. On the other hand, it is also possible to reduce the amount of catalyst.
*4 honeycombs
beehives
・beehive-like structure in which flat surfaces can be laid out with no gaps without compromising strength, creating a large hive with as little material as possible.
・Honeycomb (as a catalyst):A cylindrical structure made using a honeycomb structure to detoxify harmful components contained in exhaust gas through a chemical reaction.

*5 catalyst promoter
A substance that promotes the catalytic action of a catalyst
*6 We developed Ceria Zirconia in 2013. It works as a catalyst promoter
Professor Ozawa is the first person in the world who developed Ceria Zirconia, the key material for automotive catalyst, and has been the leader of its development. He also developed new materials in the joint development with Noritake Co., Ltd. and Nagoya Institute of Technology. The title of the NEDO project is “The Development of the Technique and the Alternative Material for the Cerium Usage Reduction Technology for Exhaust Gases and the Development of the Alternative Technique for the Cerium Usage Reduction Technology as the Catalyst for Cleaning Exhaust Gases.
*7 Cohesion
The phenomenon of tiny particulates and particulate matter in gas or liquid gathering to become larger or solidified
*8 micrometers
micrometer: the unit of length. One thousandth of one millimeter shown as μm
Text extracted from "IMaSS NEWS Vol. 07" Special Issue, IMaSS Publicity Committee (T. Miwa and M. Konishi)
[English translation by Shozo Ikuta]



