Nakanishi & Hasegawa Lab makes use of the sol-gel reaction, a liquid phase process, to study a wide range of porous materials from inorganic ceramics*2 and organic polymers*3 to organic-inorganic hybrids. Lab members’ interests extend widely from the precise control of pore structures to application to everyday products. Our interview covered what all this implies and how they are fascinated by the research on porous materials.
| How he got involved in this research |
▶You are working with porous materials, but have you been doing this research all along?
Nakanishi: I was studying physical chemistry at university up until the middle of my doctoral program. I was investigating and measuring a transport phenomenon called diffuse permeation, observing how small molecules such as those of gases and vapors pass through thin polymer membranes. It was an unglamorous research study, but that time was the dawn of the technology of gas separation using membranes. Nowadays, membranes that allow only oxygen to pass through are common. However, at that time, the fundamentals of this technology were just starting to be established.
▶When did you start working with inorganic materials?
Nakanishi: One day, Professor Naohiro Soga who was working in a completely different field, the field of inorganic materials, suddenly said to me, “There’s a vacant research associate position. You’ve worked with polymers, right?” He told me that a new method for synthesizing inorganic materials called the sol–gel method*4 had been developed for the last 20 years or so and that the process of joining together the molecules in a solution to form a material through a polymerization*5 reaction was something similar to my research. He suggested that I could find new possibilities even if the starting materials are slightly different between organic and inorganic materials.
▶Have you been working on it until now?
Nakanishi: Yes, but I was very lucky because on the floor above me at the time was Professor Takeji Hashimoto. He was leading the world in the research of patterns with regular shapes and sizes, which are formed when polymers that have been mixed are separated. I had a model nearby to help me understand the results of my experiments. I observed the materials I had made under an electron microscope and thought that they looked like patterns formed by polymers. That’s why I came up with the bold hypothesis, “What happens in polymers also happens in the solutions prepared for producing ceramics from gel”.

Professor Kazuki NAKANISHI
1986: Left the doctoral program at the Graduate School of Engineering, Kyoto University, and was appointed as a research associate at the Department of Industrial Chemistry, Faculty of Engineering, at the same university
1991 Received a doctorate in engineering from Kyoto University
2019 Appointed as a professor at the Institute for Materials and Systems Research, Nagoya University (also working at the Institute for Integrated Cell-Material Sciences, iCeMS, at Kyoto University Institute for Advanced Study), after working as an associate professor at the Graduate School of Engineering and the Graduate School of Science at Kyoto University
●Hobbies and passions: Traveling abroad for business trips, tinkering with computers, enjoying good food and drinks in moderation, and singing in a choir
| Fascination with research |
▶Would you tell us what is exciting about your research?
Nakanishi: I gradually came to understand the real fun of my research as I continued, but at first, I was just amused to see the beautiful patterns formed. When you look through a microscope, the image is first blurry, but then it comes into focus and you can clearly see what is there. That’s what makes me happy. My father, who was also a researcher (studying shape memory alloys), used to look at the images of metal under a microscope and say, “It’s like a beautiful woman smiling at me”, when he saw the phase transition*6 pattern he was expecting. I can understand now what he meant then.
▶I’m sure it’s so fun if you can observe the changes you expect to see.
Nakanishi: What looks beautiful to the eye usually has good properties too. It’s interesting that something good will happen if you can make something well-ordered. That may be also true for researchers who are studying crystals in other fields. Sometimes, however, something disordered is more interesting. I learned about disorder in a laboratory at Kyoto University where the main research theme was glass. Glass is amorphous*7 like liquid. Even though it is a solid material, the molecules in glass are not arranged in order like those in crystals. Glass has various optical applications when dyes and ions are added. That is interesting in its own way.
▶Do you aim to control the pore structures so that they are well-ordered?
Nakanishi: In my case, I control the pore structures by using a change (phase transition*8) that occurs before a certain shape is formed. Everything is mixed and then gradually separated into different components, such as components A and B. It’s like a separate-component-type salad dressing. During this process, a three-dimensional pattern is formed by the two phases intertwined with one another. I adjust the initial composition and control the temperature and catalyst conditions so that the gel hardens through polymerization at that point.
| Practical applications |
▶Are there any porous materials that have been put to practical use??
Nakanishi: In junior high school, you may learn about paper chromatography, which is used in an experiment to separate water colored with ink or flower juice into different components. Paper is made from intertwined cellulose fibers, and the gaps between the fibers form pores that allow water to pass through. When you drop water onto paper, the capillary force (capillary action) draws the water into the gaps between the fibers. Because the moving speed of each component differs depending on its affinity (degree of attraction) to the cellulose surface, the aqueous solution is separated to form a band-like pattern while moving through the gaps in the fibers.
The method we worked on was a more advanced version of this chromatography, called high-performance liquid chromatography (HPLC). It is widely used in analytical chemistry and the separation and refinement of medicines. Liquid chromatography is a method using a separation tube called a column*9 packed tightly with fine grains of silica gel with many small pores. You can analyze a liquid by making it pass through the column and observing the order in which the components come out. When we replaced the grains packed in this column with a rod-shaped porous material with controlled pores that we developed, the speed of analysis increased about 10 times even though the same silica gel was used.

Brochure of HPLC columns when they were launched
▶Was it put to practical use?
Nakanishi: Yes. It was a long time ago. We started producing this silica gel in 1987, and by around 1993, we had basically worked out how to control its structure. Around that time, we started a project to industrialize this material as an HPLC column, working with researchers in Kyoto and a German company. It took seven years to bring it to market, but it was launched on the world market in 2000. That was the most widely used product we have developed. As I mentioned previously, we were pursuing something beautiful and we happened to find a good use for it.
| Hobbies |
▶What did you like to do when you were a child?
Nakanishi: I used to read books at home, but my mother often told me to play outside. My mother taught piano at home. I also played the piano, but I quit it when I entered junior high school because I didn’t like being forced to play. Then, I was told to join a tennis club to do some exercise at junior high school. When I entered high school, I joined the brass band club and played the trumpet because I wanted to play music again. At university, I was in the orchestra for a little while and then started joining a chorus.
▶So, you come from a musical family.
Nakanishi: I resisted it when I was a child and quit playing the piano, but my sons are the opposite and are so into music that I don’t know what to do with them (the eldest son plays the piano and the second son plays the violin). They took a break from university and graduate school (the eldest son studied electrochemistry and the second son studied forest science) to study music abroad, so I’m worried about what kind of work they’ll be able to do in the future. But when I think about what I really like, I realize that I enjoy finding something with my own efforts. This attitude may have been passed down to my sons.
▶For future researchers (high school students)
Nakanishi: I think it’s better to do what you like as much as possible rather than just studying for exams. I’m sure your preparatory school teachers will tell you to focus on your weak subjects, but it’s hard to cut back on doing what you like in order to do something you don’t like. The subjects you find interesting in high school will be connected to your learning in the university and your career after that. I hope you will live your life taking on challenges, including studying abroad if you have a chance.
| How he became a researcher |
▶Did you always want to become a researcher, Professor Hasegawa?
Hasegawa: Like Professor Nakanishi, my father was also a researcher, and from a young age, I wanted to become a researcher, too. However, I entered the faculty of science because I was hesitant to choose the same fields as my father (engineering and polymer physics). I had no intention of staying at university but was thinking of finding a job in a company. Moreover, when I entered university, I was thinking of going into biological sciences. I’ve always liked plants, so I wanted to study medicinal botany and vegetation. I attended many biology lectures. But when I was in my third year, I heard at a briefing session for each major that if I went into the biology major, I would have to go on to a doctorate… and I thought “It’s no good if there are no jobs in companies”. (This was what people said at that time. It does not mean that this is generally the case.)
▶So, you decided to study chemistry?
Hasegawa: I changed my course of study at short notice to chemistry, although I had almost no credits in chemistry-related subjects in general education let alone specialized subjects (lol). I joined an organic synthesis chemistry laboratory in my fourth year because I had studied biochemistry and organic chemistry, but it was not my thing at all. I decided to join a laboratory that seemed most suitable for me at graduate school regardless of the field. When I was looking for one, I heard about the research life of my friends in an inorganic-chemistry-related field. It really sounded ideal, so I studied hard for the graduate school entrance exam in order to transfer to their laboratory. When I joined the laboratory for my master’s course, there was Associate Professor Nakanishi. It was an inorganic ceramics laboratory. However, among the research themes that were first presented to me, there was one on organic polymers. I thought I could do this even though I was not familiar with inorganic chemistry, so I chose the theme of making porous resins using a reaction called living radical polymerization. At first, I was planning to do it for just the two years of my master’s course and then get a job in a company. However, I found myself enjoying research as I continued.
▶What was it that made you enjoy it?
Hasegawa: The theme of this research was to observe using an electron microscope whether a desired pore structure was formed. After many failures, one day, when I was adjusting the focus, the beautiful pattern I had wanted to create suddenly appeared. I was so happy with this sense of achievement. After that, I just tried to create pore structures with various substances while trying different polymerization systems. When I was able to create a pore structure with one substance, then I tried with another substance. I was completely absorbed in it before I knew it.

Designated Associate Professor George HASEGAWA
2012 Completed the doctoral program at the Graduate School of Science, Kyoto University (Doctor of Science), and started working as a postdoctoral fellow of Japan Society for the Promotion of Science (JSPS) at the Graduate School of Engineering, Kyoto University
2015 Assistant professor at the Institute of Scientific and Industrial Research, Osaka University (term-limited)
2016 Assistant professor at the Graduate School of Engineering, Kyushu University
2019 Designated Associate Professor at the Institute of Materials and Systems for Sustainability (IMaSS), Nagoya University
2021 FOREST researcher (first generation), the Japan Science and Technology Agency (JST)
●Hobbies and passions:
Growing plants (I’m currently growing them in my room and on my balcony, but I’d really like to have a large vegetable garden). Drinking (I have a drink almost every night and also go to sake festivals and sake brewery openings).
▶What kind of materials did you make?
Hasegawa: At first, I made materials that were like relatives of polystyrene. Then, I made polyacrylamide (the same material as the electrophoretic gel, which is often used in biological sciences to separate proteins) and methacrylate.
▶So, you worked on a variety of materials. Professor Nakanishi told us that your doctoral thesis contained 15 papers.
Hasegawa: I just worked on any substances that would gel. I only worked on organic polymers in the first year of my master’s program, but I also started working on organic–inorganic hybrid systems halfway through my second year. I thought that classroom learning and experiments were two different things. I gradually started to work on ceramics too, starting with porous titania (titanium dioxide), and I ended up enrolling in the doctoral program.
| Desire for manufacturing |
▶You have been doing research in a wide range of fields.
Hasegawa: At that time, I had the desire to use those materials for something, so I started by baking a polymer to turn it into carbon and using it as electrodes. I thought it might be used as electrodes for electric double-layer capacitors*10 and lithium-ion secondary batteries. As we had no experience in electrochemical measurements, I went to a group that could perform such measurements once or twice a week to learn how to do it, and I conducted experiments. In the meantime, electrochemistry became one of my areas of expertise.
▶The starting point of manufacturing is the “desire to use it for something”, isn’t it?
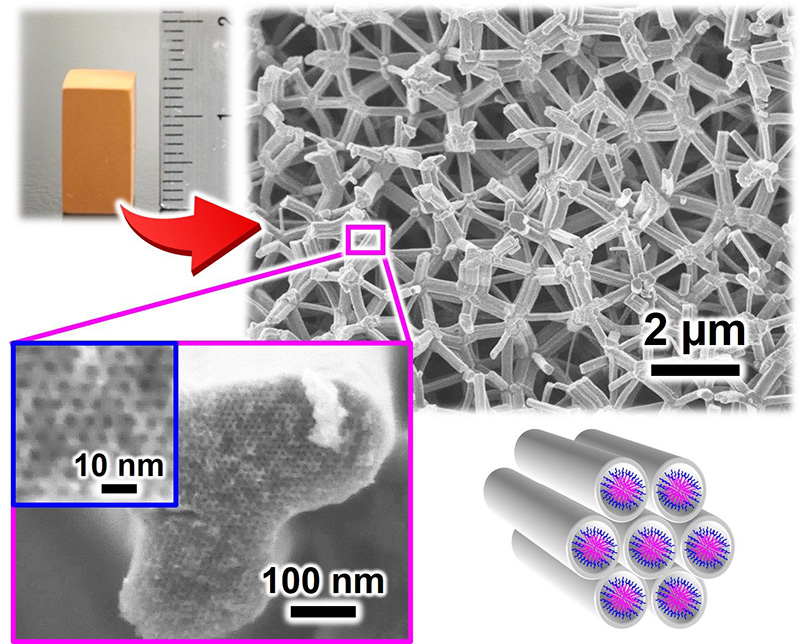
Hasegawa: My current research topics are quite varied, but there is one I’ve been working on since I was a student, which is porous monoliths*11. They are made by creating pores in a centimeter-scale mass through phase separation. Powders all look the same even if they are different substances, but if it’s a mass, you can pick it up and touch it with your hands. As I love manufacturing, I really feel a sense of achievement from “creating” something.
▶The theme you are currently working on is “rigid and flexible materials”, right?
Hasegawa: Yes. Since the materials we developed are 90% pores, they are very light. Even if it is compressed to 80%, it does not break but deforms and recovers. Yet, it is harder than cork stoppers. Recently, I was told that it might be a good material for face guards for professional sports, but we are still looking for more uses of this material.
▶Message for high school students
Hasegawa: I’m a materials chemistry researcher, so I hope that high school students will be interested in manufacturing through chemistry and go to this field with a goal or intention, such as what they want to do at university. However, I can’t say anything arrogant because I was not like that at all. So, I think the best thing for high school students is to be interested in everything and to take on new challenges. Even if you think something might not be for you, you may later find it interesting and get absorbed in it, just like I did.
Glossary
*1 Porous materials
Materials with many pores. Examples include silica gel, porous glass, activated carbon, and zeolite. All of these materials have a large surface area per unit volume and can highly absorb molecules and ions of gases and liquids.(↑ Return to the text)
*2 Inorganic ceramics
Inorganic compounds such as metal oxides, carbides, and nitrides.(↑ Return to the text)
*3 Organic polymers
A general term for organic substances with a molecular weight of 10,000 or more, which are composed of carbon as the main framework and other elements such as oxygen, hydrogen, and nitrogen. (Polymers that do not contain carbon are inorganic polymers.) Organic polymers are also simply called polymers. Plastics and resins are moldable materials made by kneading compounding agents (e.g., additives, colorants, reinforcing agents, and other polymers) into polymers.(↑ Return to the text)
*4 Sol–gel method (reaction)
An inorganic or organic metallic salt solution is transformed into a colloidal solution (sol) through hydrolysis and condensation polymerization reactions. Then, the reactions are further accelerated to form a solid (gel) that has lost its fluidity. The sol–gel method is a process of producing glass and ceramics by heat-treating this gel.
*Polycondensation is a type of polymerization reaction in which multiple compounds combine (condense) while small molecules such as water (H₂O) are removed from molecules of each compound and are then connected in a chain-like manner to form a polymer (polymerization).
*A colloidal solution is a solution in which colloidal particles with a diameter of about 10-5–10-7 cm are dispersed in liquid.
*Gel is a state in which a solution in the sol state loses its fluidity owing to viscosity and becomes a solid (e.g., konnyaku and jelly).
(↑ Return to the text)
*5 Polymerization
The process of small molecules combining to form a large molecule or a polymer. The small molecules that are the starting materials are called monomers, whereas the large molecule that is formed as a result of polymerization is called a polymer.(↑ Return to the text)
*6 Phase transition
The complete change in the state of a substance due to a physical boundary, for example, when water, which is liquid at room temperature, becomes ice (i.e., a solid) at 0 °C or below and becomes water vapor (i.e., gas) above 100°C.(↑ Return to the text)
*7 Amorphous
Substances in which atoms or ions are not orderly arranged, unlike crystals.(↑ Return to the text)
*8 Phase separation
Separation of a substance that was in a single phase into multiple phases due to changes in temperature and pressure. This is a type of phase transition phenomenon.
For example, the state in which ice and water, and water and water vapor coexist during a phase transition.(↑ Return to the text)
*9 Column
A tube, usually made of metal, about 5 mm in diameter and 10–20 cm in length, and filled with particles such as silica gel that act as a separation medium. When a sample solution is poured through the gaps between the particles, solutes with a high affinity for the surface of the separation medium remain in the column for a long time, while those with a low affinity are quickly eluted. It is therefore possible to separate multiple solutes on the basis of the time it takes for each solute to come out of the column.(↑ Return to the text)
*10 Electric double-layer capacitors
Power storage devices are classified as capacitors. They have excellent power density and suffer less performance degradation due to repeated charging and discharging at high currents.(↑ Return to the text)
*11 Porous monoliths
Monolithic porous materials with a characteristic structure consisting of interconnected micrometer-scale mesh-like frameworks. They look like chalk. However, when observed under an electron microscope, their structure consists of interconnected frameworks like a jungle gym. In addition, the frameworks have nanoscale pores called mesopores. Porous monoliths are light because of their high porosity. They also have a very large specific surface area because of the large number of nanoscale pores.(↑ Return to the text)